Redox



Redox reducing agents are electron donors oxidising agents are electron acceptors oxidation is the process of electron loss: Zn Zn2+ + 2eIt involves an increase in oxidation number reduction is the process of electron gain: Cl2 + 2e- 2ClIt involves a decrease in oxidation number Redox equations and half equations Br2 (aq) + 2I- (aq) I 2 (aq) + 2 Br- (aq) Br2 (aq) + 2e- + 2 Br- (aq) 2I- (aq) I 2 (aq) + 2 eBr has reduced as it has gained electrons I has oxidised as it has lost electrons When naming oxidising and reducing agents always refer to full name of substance and not just name of element The oxidising agent is Bromine water . It is an electron acceptor The reducing agent is the Iodide ion. It is an electron donor An oxidising agent (or oxidant) is the species that causes another element to oxidise. It is itself reduced in the reaction A reducing agent (or reductant) is the species that causes another element reduce. It is itself oxidised in the reaction. A reduction half equation only shows the parts of a chemical equation involved in reduction The electrons are on the left An oxidation half equation only shows the parts of a chemical equation involved in oxidation The electrons are on the right. Balancing Redox equations Writing half equations 1. Work out oxidation numbers for element being oxidised/ reduced Zn Zn2+ Zn changes from 0 to +2 2. Add electrons equal to the change in oxidation number For reduction add e’s to reactants For oxidation add e’s to products Zn Zn2+ + 2e- 3. check to see that the sum of the charges on the reactant side equals the sum of the charges on the product side 0 +2 –2 =0 -1 + 8 -5 = +2 More complex Half equations If the substance that is being oxidised or reduced contains a varying amount of O (eg MnO4 – Mn2+ ) then the half equations are balanced by adding H+ , OHions and H2O. In acidic conditions use H+ and H2O Example: Write the half equation for the change MnO4 – Mn2+ 1. Balance the change in O.N. with electrons MnO4 – + 5e- Mn Mn changes from +7 to +2 2+ Add 5 electrons to reactants 2. Add H2O in products to balance O’s in MnO4 – MnO4 – + 5e- Mn2+ + 4H2O 3. Add H+ in reactants to balance H’s in H2O MnO4 – + 8H+ + 5e- Mn2+ + 4H2O 4. check to see that the sum of the charges on the reactant side equals the sum of the charges on the product side Combining half equations To make a full redox equation combine a reduction half equation with a oxidation half equation To combine two half equations there must be equal numbers of electrons in the two half equations so that the electrons cancel out Reduction MnO4 – + 8 H+ + 5 e- → Mn2+ + 4 H2O Oxidation C2O4 2- → 2 CO2 + 2 ex2 x5 Multiply the half equations to get equal electrons 2MnO4 – + 16 H+ + 5C2O4 2- → 2Mn2+ + 10 CO2 + 8 H2O Add half equations together and cancel electrons -4 + 4 = 0 Example: Write the half equation for the change SO4 2- SO2 1. Balance the change in O.N. with electrons SO4 2- + 2e- SO2 S changes from +6 to +4 Add 2 electrons to reactants 2. Add H2O in products to balance O’s in SO4 2- 3. Add H+ in reactants to balance H’s in H2O SO4 2- + 4H+ + 2e- SO2 + 2H2O 4. check to see that the sum of the charges on the reactant side equals the sum of the charges on the product side 0 SO4 2- + 2e- SO2 + 2H2O Reduction SO4 2- + 10H+ + 8e- H2S+ 4H2O Oxidation 2I- → I 2 + 2 ex4 Multiply the half equations to get equal electrons 8I- + SO4 2- + 10H+ H2S+ 4I2 + 4H2O Add half equations together and cancel electrons
/
~
~
~
/
5.2.3 Redox and electrode potentials

Redox (a) explanation and use of the terms oxidising agent and reducing agent (see also 2.1.5 Redox) (b) construction of redox equations using halfequations and oxidation numbers M0.2 (c) interpretation and prediction of reactions involving electron transfer
Redox titrations



Manganate redox titration The redox titration between Fe2+ with MnO4 – (purple) is a very common exercise. This titration is self indicating because of the significant colour change from reactant to product MnO4 – (aq) + 8H+ (aq) + 5Fe2+ (aq) Mn2+ (aq) + 4H2O (l) + 5Fe3+ (aq) Purple colourless Choosing correct acid for manganate titrations. The acid is needed to supply the 8H+ ions. Some acids are not suitable as they set up alternative redox reactions and hence make the titration readings inaccurate. Only use dilute sulphuric acid for manganate titration Insufficient volumes of sulphuric acid will mean the solution is not acidic enough and MnO2 will be produced instead of Mn2+ MnO4 – (aq) + 4H+ (aq) + 3e- MnO2 (s) + 2H2O The brown MnO2 will mask the colour change and lead to a greater (inaccurate) volume of Manganate being used in the titration Using a weak acid like ethanoic acid would have the same effect as it cannot supply the large amount of hydrogen ions needed (8H+ ) It cannot be conc HCl as the Clions would be oxidised to Cl2 by MnO4 – as the Eo MnO4 – /Mn2+ > Eo Cl2 /ClMnO4 – (aq) + 8H+ (aq) + 5e– Mn2+ (aq) + 4H2O(l) E+1.51V Cl2 (aq) +2e– 2Cl– (aq) E +1.36V This would lead to a greater volume of manganate being used and poisonous Cl2 being produced It cannot be nitric acid as it is an oxidising agent. It oxidises Fe2+ to Fe3+ as Eo NO3 – /HNO2> Eo Fe3+/Fe2+ NO3 – (aq) + 3H+ (aq) + 2e– HNO2 (aq) + H2O(l) Eo +0.94V Fe3+ (aq)+e– Fe2+ (aq) Eo +0.77 V This would lead to a smaller volume of manganate being used The purple colour of manganate can make it difficult to see the bottom of the meniscus in the burette. If the manganate is in the burette then the end point of the titration will be the first permanent pink colour. Colourless purple N Goalby chemrevise.org Thiosulphate redox titration The redox titration between I2 and thiosulphate S2O3 2- is a common exercise. 2S2O3 2-(aq) + I2 (aq) 2I- (aq) + S4O6 2-(aq) yellow/brown sol colourless sol A starch indicator is added near the end point when the iodine fades a pale yellow to emphasise it. With starch added the colour change is from blue/black to colourless R. Other useful manganate titrations With hydrogen peroxide Ox H2O2 O2 + 2H+ + 2eRed MnO4 – (aq) + 8H+ (aq) + 5e- Mn2+ (aq) + 4H2O Overall 2MnO4 – (aq) + 6H+ (aq) + 5H2O2 5O2 + 2Mn2+ (aq) + 8H2O Ox C2O4 2- 2CO2 + 2eRed MnO4 – (aq) + 8H+ (aq) + 5e- Mn2+ (aq) + 4H2O Overall 2MnO4 – (aq) + 16H+ (aq) + 5C2O4 2-(aq) 10CO2 (g) + 2Mn2+(aq) + 8H2O(l) With ethanedioate With Iron (II) ethanedioate both the Fe2+ and the C2O4 2- react with the MnO4 – 1MnO4 – reacts with 5Fe2+ and 2 MnO4 – reacts with 5C2O4 2- MnO4 – (aq) + 8H+ (aq) + 5Fe2+ Mn2+ (aq) + 4H2O + 5Fe3+ 2MnO4 – (aq) + 16H+ (aq) + 5C2O4 2- 10CO2 + 2Mn2+ (aq) + 8H2O So overall 3MnO4 – (aq) + 24H+ (aq) + 5FeC2O4 10CO2 + 3Mn2+ (aq) + 5Fe3+ + 12H2O So overall the ratio is 3 MnO4 – to 5 FeC2O4 The reaction between MnO4 – and C2O4 2- is slow to begin with (as the reaction is between two negative ions) To do as a titration the conical flask can be heated to 60o C to speed up the initial reaction. N Goalby chemrevise.org A 1.412 g sample of impure FeC2O4 .2H2O was dissolved in an excess of dilute sulphuric acid and made up to 250 cm3 of solution. 25.0 cm3 of this solution decolourised 23.45 cm3 of a 0.0189 mol dm–3 solution of potassium manganate(VII). What is the the percentage by mass of FeC2O4 .2H2O in the original sample? Step1 : find moles of KMnO4 moles = conc x vol 0.0189 x 23.45/1000 = 4.43×10-4 mol Step 2 : using balanced equation find moles FeC2O4 .2H2O in 25cm3 = moles of KMnO4 x 5/3 (see above for ratio) = 7.39×10-4 mol Step 3 : find moles FeC2O4 .2H2O in 250 cm3 = 7.39×10-4 mol x 10 = 7.39×10-3 mol Step 4 : find mass of FeC2O4 .2H2O in 7.39×10-3 mol mass= moles x Mr = 7.39×10-3 x 179.8 = 1.33g Step 5 ; find % mass %mass = 1.33/1.412 x100 = 94.1%. be able to perform calculations for these titrations and for others when the reductant and its oxidation product are given. A 2.41g nail made from an alloy containing iron is dissolved in 100cm3 acid. The solution formed contains Fe(II) ions. 10cm3 portions of this solution are titrated with potassium manganate (VII) solution of 0.02M. 9.80cm3 of KMnO4 were needed to react with the solution containing the iron. What is the percentage of Iron by mass in the nail? Manganate titration example MnO4 – (aq) + 8H+ (aq) + 5Fe2+ Mn2+ (aq) + 4H2O + 5Fe3+ Step1 : find moles of KMnO4 moles = conc x vol 0.02 x 9.8/1000 = 1.96×10-4 mol Step 2 : using balanced equation find moles Fe2+ in 10cm3 = moles of KMnO4 x 5 = 9.8×10-4 mol Step 3 : find moles Fe2+ in 100cm3 = 9.8×10-4 mol x 10 = 9.8×10-3 mol Step 4 : find mass of Fe in 9.8×10-3 mol mass= moles x RAM = 9.8×10-3 x 55.8 = 0.547g Step 5 ; find % mass %mass = 0.547/2.41 x100 = 22.6%
/
~
~
~
/
5.2.3 Redox and electrode potentials

Redox titrations (d) the techniques and procedures used when carrying out redox titrations including those involving Fe2+/MnO4 – and I2/S2O3 2− (see also 2.1.5 e–f) HSW4 Opportunities to carry out experimental and investigative work. (e) structured and non-structured titration calculations, based on experimental results of redox titrations involving: (i) Fe2+/MnO4 – and I2/S2O3 2− (ii) non-familiar redox systems M0.1, M0.2, M0.4, M1.1, M1.2, M2.2, M2.3, M2.4 Non-structured titration calculations could be examined in the context of both acid–base and redox titrations (see also 2.1.4 d–e).
Electrochemical cells


Electrochemical cells •A cell has two half–cells. •The two half cells have to be connected with a salt bridge. •Simple half cells will consist of a metal (acts an electrode) and a solution of a compound containing that metal (eg Cu and CuSO4 ). •These two half cells will produce a small voltage if connected into a circuit. (i.e. become a Battery or cell). Salt Bridge The salt bridge is used to connect up the circuit. The free moving ions conduct the charge. A salt bridge is usually made from a piece of filter paper (or material) soaked in a salt solution, usually Potassium Nitrate. The salt should be unreactive with the electodes and electrode solutions.. E.g. potassium chloride would not be suitable for copper systems as Chloride ions can form complexes with copper ions. A wire is not used because the metal wire would set up its own electrode system with the solutions. Why does a voltage form? In the cell pictured above When connected together the zinc half-cell has more of a tendency to oxidise to the Zn2+ ion and release electrons than the copper half-cell. (Zn Zn2+ + 2e- ) More electrons will therefore build up on the zinc electrode than the copper electrode. A potential difference is created between the two electrodes. The zinc strip is the negative terminal and the copper strip is the positive terminal. This potential difference is measured with a high resistance voltmeter, and is given the symbol E. The E for the above cell is E= +1.1V. Why use a High resistance voltmeter? The voltmeter needs to be of very high resistance to stop the current from flowing in the circuit. In this state it is possible to measure the maximum possible potential difference (E). The reactions will not be occurring because the very high resistance voltmeter stops the current from flowing. What happens if current is allowed to flow? If the voltmeter is removed and replaced with a bulb or if the circuit is short circuited, a current flows. The reactions will then occur separately at each electrode. The voltage will fall to zero as the reactants are used up. The most positive electrode will always undergo reduction. Cu2+ (aq) + 2e- Cu(s) (positive as electrons are used up) The most negative electrode will always undergo oxidation. Zn(s) Zn2+ (aq) + 2e- (negative as electrons are given off) N Goalby chemrevise.org Zinc electrode copper electrode 1M zinc sulphate solution 1M copper sulphate solution Salt bridge Electron flow
/
~
~
~
/
5.2.3 Redox and electrode potentials
Not explicitly stated but required for subsequent concepts
Measuring standard electrode potential PAG8
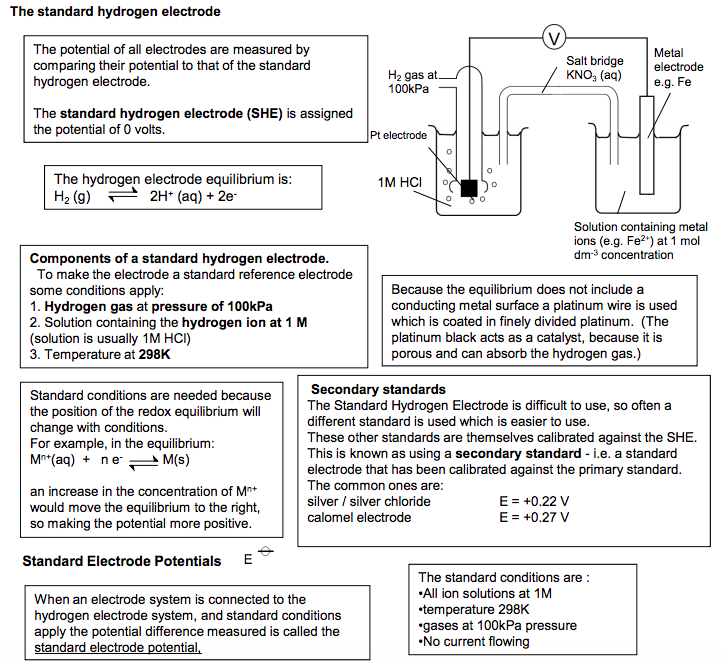

Measuring the electrode potential of a cell •It is not possible to measure the absolute potential of a half electrode on its own. It is only possible to measure the potential difference between two electrodes. • To measure it, it has to be connected to another half-cell of known potential, and the potential difference between the two half-cells measured. •by convention we can assign a relative potential to each electrode by linking it to a reference electrode (hydrogen electrode), which is given a potential of zero Volts 6 The standard hydrogen electrode The potential of all electrodes are measured by comparing their potential to that of the standard hydrogen electrode. The standard hydrogen electrode (SHE) is assigned the potential of 0 volts. The hydrogen electrode equilibrium is: H2 (g) 2H+ (aq) + 2eBecause the equilibrium does not include a conducting metal surface a platinum wire is used which is coated in finely divided platinum. (The platinum black acts as a catalyst, because it is porous and can absorb the hydrogen gas.) Components of a standard hydrogen electrode. To make the electrode a standard reference electrode some conditions apply: 1. Hydrogen gas at pressure of 100kPa 2. Solution containing the hydrogen ion at 1 M (solution is usually 1M HCl) 3. Temperature at 298K Secondary standards The Standard Hydrogen Electrode is difficult to use, so often a different standard is used which is easier to use. These other standards are themselves calibrated against the SHE. This is known as using a secondary standard – i.e. a standard electrode that has been calibrated against the primary standard. The common ones are: silver / silver chloride E = +0.22 V calomel electrode E = +0.27 V Standard conditions are needed because the position of the redox equilibrium will change with conditions. For example, in the equilibrium: Mn+(aq) + n e- M(s) an increase in the concentration of Mn+ would move the equilibrium to the right, so making the potential more positive. Standard Electrode Potentials The standard conditions are : •All ion solutions at 1M •temperature 298K •gases at 100kPa pressure •No current flowing When an electrode system is connected to the hydrogen electrode system, and standard conditions apply the potential difference measured is called the standard electrode potential, E Standard electrode potentials are found in data books and are quoted as Li+ (aq) | Li (s) E= -3.03V more oxidised form on left They may also be quoted as half equations Li+ (aq) + e- Li (s) E= -3.03V but again the more oxidised form is on the left Pt electrode Pt electrode 1M HCl 1M Fe2+ and 1M Fe3+ Salt bridge KNO3 (aq) H2 gas at 100kPa Solution containing metal ions (e.g. Fe2+) at 1 mol dm-3 concentration Metal electrode e.g. Fe H2 gas at 100kPa 1M HCl Pt electrode Salt bridge KNO3 (aq) N Goalby chemrevise.org Note: in the electrode system containing two solutions it is necessary to use a platinum electrode and both solutions must be of a 1M concentration.
/
~
~
~
/
5.2.3 Redox and electrode potentials

Electrode potentials (f) use of the term standard electrode (redox) potential, E o including its measurement using a hydrogen electrode E o data will be provided on examination papers. (g) the techniques and procedures used for the measurement of cell potentials of: (i) metals or non-metals in contact with their ions in aqueous solution (ii) ions of the same element in different oxidation states in contact with a Pt electrode For measurement of standard cell potentials, ions of the same element can have concentrations of 1 mol dm–3 or be equimolar. PAG8 HSW4 Opportunities to carry out experimental and investigative work. 8 Electrochemical cells • Set up of electrochemical cells and measurement of
voltages
The effect of concentration on the cell
potential of an electrochemical cell
5.2.3(g)
voltages
The effect of concentration on the cell
potential of an electrochemical cell
5.2.3(g)
Calculating standard cell potential




Using electrode potentials The most useful application of electrode potentials is to show the direction of spontaneous change for redox reactions The easiest way to use electrode potentials is as follows: For any two half equations The more negative half cell will always oxidise (go backwards) Mg2+ (aq) + 2e- Mg(s) E= -2.37V Cu2+ (aq) + 2e- Cu (s) E = +0.34V The more positive half cell will always reduce (go forwards) The reaction would be Mg + Cu2+ Cu + Mg 2+ If we want to work out the Ecell that corresponds to this spontaneous change then use Ecell = Ered – Eox A spontaneous change will always have a positive Ecell Zn2+(aq) + 2e- Zn(s) E= – 0.76V Fe2+(aq) + 2e- Fe(s) E= -0.44V The more positive electrode will reduce and go from left to right Fe2+ (aq) +2e- Fe(s) Electrons arrive at this electrode and are absorbed (gained) To get the full equation of the reaction add the two half reactions together, cancelling out the electrons. Zn + Fe2+ Fe + Zn2+ The most negative electrode will oxidise and go from right to left The half equation is therefore Zn(s) Zn2+ (aq) +2eElectrons are given off (lost) and travel to positive electrode Using series of standard electrode potentials Li+ + e- Li -3.03V Mn2+ + 2e- Mn -1.19V 2H+ + 2e- H2 0V Ag+ + e- Ag +0.8V F2 + 2e- 2F- +2.87 As more +ve increasing tendency for species on left to reduce, and act as oxidising agents As more -ve increasing tendency for species on right to oxidise, and act as reducing agents oxidation reduction Most strong reducing agents found here Most strong oxidising agents found here The most powerful reducing agents will be found at the most negative end of the series on the right (ie the one with the lower oxidation number) The most powerful oxidising agents will be found at the most positive end of the series on the left (ie the one with the higher oxidation number) If we want to work out the Ecell from two standard electrode potentials then use Ecell = Ered – Eox N Goalby chemrevise.org 8 O2 (g) + 4H+ (aq) + 4e– → 2H2O(I) Eo+1.23V F2 (g) + 2e– → 2F– (aq) Eo +2.87V Use electrode data to explain why fluorine reacts with water. Write an equation for the reaction that occurs. Cl2 (aq) + 2e– → 2Cl– (aq) Eo+1.36V 2HOCl(aq) + 2H+ (aq) + 2e– → Cl2 (aq) + 2H2O(I) Eo+1.64V H2O2 (aq) + 2H+ (aq) + 2e– → 2H2O(I) Eo +1.77V O2 (g) + 4H+ (aq) + 4e– → 2H2O(I) Eo +1.23V Use data from the table to explain why chlorine should undergo a redox reaction with water. Write an equation for this reaction. Use the half-equations to explain in terms of oxidation states what happens to hydrogen peroxide when it is reduced. H2O2 (aq) + 2H+ (aq) + 2e– → 2H2O(I) Eo+1.77V O2 (g) + 2H+ (aq) + 2e– → H2O2 (aq) Eo +0.68V Example 1 First apply idea that more positive Eo will reduce (go forward) and more negative Eo will oxidise (go backwards) reduce oxidise Explanation to write As Eo F2 /F- > Eo O2 /H2O, F2 will oxidise H2O to O2 Equation 2F2 (g) + 2H2O(I) → 4F– (aq) + O2 (g) + 4H+ (aq) Can also work out Ecell and quote it as part of your answer Ecell = Ered – Eox = 2.87-1.23 =1.64V Remember to cancel out electrons in full equation Example 2 First select relevant half equations by considering the Eo values and applying the idea that more positive Eo will reduce (go forward) and more negative Eo will oxidise (go backwards) Cl2 (aq) + 2e– → 2Cl– (aq) Eo+1.36V O2 (g) + 4H+ (aq) + 4e– → 2H2O(I) Eo +1.23V reduce oxidise Explanation to write As Eo Cl2 /Cl- > Eo O2 /H2O, Cl2 will oxidise H2O to O2 Equation 2Cl2 (g) + 2H2O(I) → 4Cl– (aq) + O2 (g) + 4H+ (aq) Fe3+ (aq) + e– → Fe2+ (aq) Eo +0.77V 2H+ (aq) + 2e– → H2 (g) Eo 0.00V Fe2+ (aq) + 2e– → Fe(s) Eo–0.44V Suggest what reactions occur, if any, when hydrogen gas is bubbled into a solution containing a mixture of iron(II) and iron(III) ions. Explain your answer. First select relevant half equations by considering the Eo values and applying the idea that more positive Eo will reduce (go forward) and more negative Eo will oxidise (go backwards) Fe3+ (aq) + e– → Fe2+ (aq) Eo +0.77V 2H+ (aq) + 2e– → H2 (g) Eo 0.00V oxidise reduce Explanation to write Fe3+ will be reduced to Fe2+ by H2 oxidising to H+ because Eo Fe3+ /Fe2+ > Eo H+ /H2 Equation 2Fe3+ (aq) + H2 (g) → 2Fe2+ (aq) + 2H+ (aq) Example 3 reduce oxidise Explanation to write As Eo H2O2 /H2O > Eo O2 /H2O2 , H2O2 disproportionates from -1 oxidation state to 0 in O2 and -2 in H2O 2H2O2 (aq) → 2H2O(I) + O2
/
~
~
~
/
5.2.3 Redox and electrode potentials
![]()
(h) calculation of a standard cell potential by combining two standard electrode potentials
Effects of conditions on cell emf

Effect of conditions on cell e.m.f The effects of changing conditions on cell e.m.f can be made by applying le chateliers principle E.m.f. is a measure of how far from equilibrium the cell reaction lies. The more positive the e.m.f the more likely the reaction is to occur. If current is allowed to flow, the cell reaction will occur and the emf will fall to zero as the reaction proceeds and the reactant concentrations drop Effect of concentration on cell e.m.f Looking at cell reaction is a straight forward application of le chatelier. So increasing concentration of ‘reactants’ would increase EMF and decreasing them would cause EMF to decrease Increasing the concentration of Fe2+ and decreasing the concentration of Zn2+ would cause Ecell to increase Effect of temperature on cell e.m.f Most Ecells are exothermic in the spontaneous direction so applying Le chatelier to a temperature rise to these would result in a decrease in Ecell. Zn2+(aq) + 2e- Zn(s) E= – 0.76V Fe2+(aq) + 2e- Fe(s) E= -0.44V Zn + Fe2+ Fe + Zn2+ E= +0.32. If the Ecell positive it indicates a reaction might occur, there is still a possibility, however, that the reaction will not occur or will occur so slowly that effectively it doesn’t happen. If the reaction has a high activation energy the reaction will not occur.
/
~
~
~
/
5.2.3 Redox and electrode potentials

(i) prediction of the feasibility of a reaction using standard cell potentials and the limitations of such predictions in terms of kinetics and concentration M0.3 HSW6 The relative effects of standard electrode potential, rate and concentration in determining feasibility of processes.
Cells

Cells Electrochemical cells can be used as a commercial source of electrical energy Cells can be non-rechargeable (irreversible), rechargeable and fuel cells You should be able to work out Ecell for given half reactions. N Goalby chemrevise.org 9 Example primary non rechargeable cells Dry Cell Zn2+(aq) + 2e- Zn(s) E = – 0.76 V 2MnO2 (s) + 2NH4 + (aq) + 2 e- → Mn2O3 (s) + 2NH3 (aq) + H2O(l) E = 0.75 V More negative half equation will oxidise Overall reaction 2MnO2 + 2NH4 ++ Zn → Mn2O3 + 2NH3 + H2O + Zn2+ Ecell =+1.51V You do not need to learn the details of these cells. Relevant cell information will be given. You should be able to convert between standard electrode potential half cells, full cell reactions and be able to calculate potentials from given data Cells are non-rechargeable when the reactions that occur with in them are non-reversible Example secondary Nickel–cadmium cells are used to power electrical equipment such as drills and shavers. They are rechargeable cells. The electrode reactions are shown below. NiO(OH) + H2O + e- Ni(OH)2 + OH– E = +0.52 V (Ni will reduce changing oxidation state from 3 to 2) Cd(OH)2 + 2e- Cd + 2OH– E = –0.88 V (Cd will oxidise changing oxidation state from 0 to 2) Overall reaction discharge 2NiO(OH) + Cd + 2H2O 2Ni(OH)2 + Cd(OH)2 E= +1.40V Ecell = E red- Eox = +0.52 – – 0.88 = + 1.40 V The overall reaction would be reversed in the recharging state 2Ni(OH)2 + Cd(OH)2 2NiO(OH) + Cd + 2H2O
/
~
~
~
/
5.2.3 Redox and electrode potentials

Storage and fuel cells (j) application of principles of electrode potentials to modern storage cells Details of storage cells and required equations will be provided. Relevant electrode potentials and other data will be supplied. HSW9 Benefits of electrochemical cells counteracted by risks from toxicity and fire from Li-based cells.
Fuel cells

Scientists in the car industry are developing fuel cell vehicles, fuelled by: (i) hydrogen gas, (ii) hydrogen-rich fuels;Fuel cell A fuel cell uses the energy from the reaction of a fuel with oxygen to create a voltage Limitations of hydrogen fuel cells (i) storing and transporting hydrogen, in terms of safety, feasibility of a pressurised liquid and a limited life cycle of a solid ‘adsorber’ or ‘absorber’ (ii) limited lifetime (requiring regular replacement and disposal) and high production costs, (iii) use of toxic chemicals in their production Advantages of Fuel cells over conventional petrol or diesel-powered vehicles (i) less pollution and less CO2 . (Pure hydrogen emits only water whilst hydrogen-rich fuels produce only small amounts of air pollutants and CO2). (ii) greater efficiency; Hydrogen Fuel cell (potassium hydroxide electrolyte) 4e- + 4H2O 2H2 +4OH- E=-0.83V 4e- + 2H2O +O2 4OH- E=+0.4V Overall reaction 2H2 + O2 2H2O E=1.23V Using standard conditions: The rate is too slow to produce an appreciable current. Higher temperatures are therefore used to increase rate but the reaction is exothermic so by applying le chatelier would mean the emf falls. A higher pressure can help counteract this 2e- + 2H+ H2 E=0V 4e- + 4H+ +O2 2H2O E=1.23V In acidic conditions these are the electrode potentials. The Ecell is the same as alkaline conditions as the overall equation is the same O2 from air H2O +heat H2 from fuel Overall 2H2 + O2 2H2O E=1.23V Fuel cells will maintain a constant voltage over time as they are continuously fed with fresh O2 and H2 so maintaining constant concentration of reactants. This differs from ordinary cells where the voltage drops over time as the reactant concentrations drop
/
~
~
~
/
5.2.3 Redox and electrode potentials

(k) explanation that a fuel cell uses the energy from the reaction of a fuel with oxygen to create a voltage and the changes that take place at each electrode. Recall of fuel cells and equations will not be required. Relevant electrode potentials and other data will be supplied.
Credits: Neil Goalby

