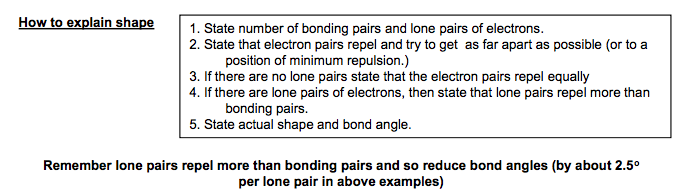

How to explain shape 1. State number of bonding pairs and lone pairs of electrons. 2. State that electron pairs repel and try to get as far apart as possible (or to a position of minimum repulsion.) 3. If there are no lone pairs state that the electron pairs repel equally 4. If there are lone pairs of electrons, then state that lone pairs repel more than bonding pairs. 5. State actual shape and bond angle. Remember lone pairs repel more than bonding pairs and so reduce bond angles (by about 2.5o per lone pair in above examples) Occasionally more complex shapes are seen that are variations of octahedral and trigonal bipyramidal where some of the bonds are replaced with lone pairs. You do not need to learn the names of these but ought to be able to work out these shapes using the method below e.g XeF4 e.g. BrF5 e.g I3 – e .g.ClF3 e.g. SF4 & IF4+ :X X X: : : X: : Xe has 8 electrons in its outer shell. 4 F’s add 4 more electrons. This makes a total of 12 electrons made up of 4 bond pairs and 2 lone pairs. The means it is a variation of the 6 bond pair shape (octahedral) Cl has 7 electrons in its outer shell. 3 F’s add 3 more electrons. This makes a total of 10 electrons made up of 3 bond pairs and 2 lone pairs. The means it is a variation of the 5 bond pair shape (trigonal bipyramidal) I has 7 electrons in its outer shell. 4 F’s add 4 more electrons. Remove one electron as positively charged. This makes a total of 10 electrons made up of 4 bond pairs and 1 lone pair. The means it is a variation of the 5 bond pair shape (trigonal bipyramidal)
/
~
~
~
/
3.1.3.5 Shapes of simple molecules and ions
Bonding pairs and lone (non-bonding) pairs of electrons as charge clouds that repel each other.
Pairs of electrons in the outer shell of atoms arrange themselves as far apart as possible to minimise repulsion.
Lone pair–lone pair repulsion is greater than lone pair–bond pair repulsion, which is greater than bond pair–bond pair repulsion.
The effect of electron pair repulsion on bond angles.
Students should be able to explain the shapes of, and bond angles in, simple molecules and ions with up to six electron pairs (including lone pairs of electrons) surrounding the central atom.


 1.3 Bonding Page 3 - 4
1.3 Bonding Page 3 - 4 Oxford Textbook Pages : 60 - 63
Oxford Textbook Pages : 60 - 63 CGP Revision Guide Pages : 28 - 29
CGP Revision Guide Pages : 28 - 29