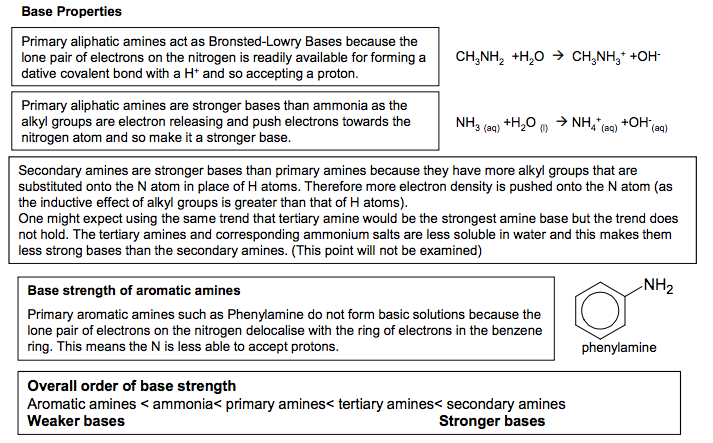
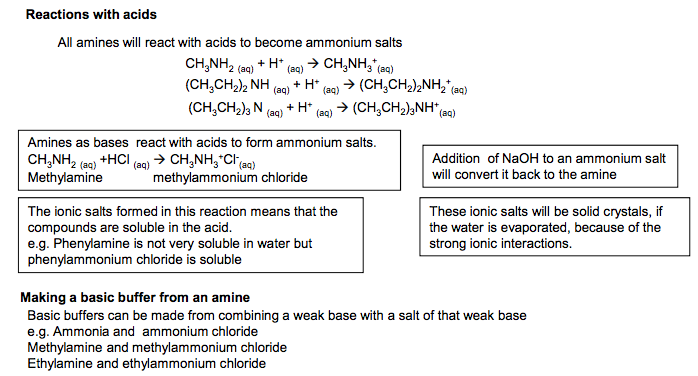
Primary aliphatic amines act as Bronsted-Lowry Bases because the lone pair of electrons on the nitrogen is readily available for forming a dative covalent bond with a H+ and so accepting a proton. Primary aliphatic amines are stronger bases than ammonia as the alkyl groups are electron releasing and push electrons towards the nitrogen atom and so make it a stronger base. Base strength of aromatic amines Primary aromatic amines such as Phenylamine do not form basic solutions because the lone pair of electrons on the nitrogen delocalise with the ring of electrons in the benzene ring. This means the N is less able to accept protons. Base Properties Amines as bases react with acids to form ammonium salts. CH3NH2 (aq) +HCl (aq) CH3NH3+Cl- (aq) Methylamine methylammonium chloride Addition of NaOH to an ammonium salt will convert it back to the amine The ionic salts formed in this reaction means that the compounds are soluble in the acid. e.g. Phenylamine is not very soluble in water but phenylammonium chloride is soluble These ionic salts will be solid crystals, if the water is evaporated, because of the strong ionic interactions. Secondary amines are stronger bases than primary amines because they have more alkyl groups that are substituted onto the N atom in place of H atoms. Therefore more electron density is pushed onto the N atom (as the inductive effect of alkyl groups is greater than that of H atoms). One might expect using the same trend that tertiary amine would be the strongest amine base but the trend does not hold. The tertiary amines and corresponding ammonium salts are less soluble in water and this makes them less strong bases than the secondary amines. (This point will not be examined) NH2 phenylamine Reactions with acids Making a basic buffer from an amine Basic buffers can be made from combining a weak base with a salt of that weak base e.g. Ammonia and ammonium chloride Methylamine and methylammonium chloride Ethylamine and ethylammonium chloride. Overall order of base strength Aromatic amines < ammonia< primary amines< tertiary amines< secondary amines Weaker bases Stronger bases
/
~
~
~
/
3.3.11.2 Base properties (A-level only)
Amines are weak bases.
The difference in base strength between ammonia, primary aliphatic and primary aromatic amines.
Students should be able to explain the difference in base strength in terms of the availability of the lone pair of electrons on the N atom.


 3.11 Amines Page 1
3.11 Amines Page 1 Oxford Textbook Pages : 442 - 443
Oxford Textbook Pages : 442 - 443 CGP Revision Guide Pages : 175
CGP Revision Guide Pages : 175











