
Alkenes are unsaturated hydrocarbons General formula is CnH2n Alkenes contain a carbon- carbon double bond somewhere in their structure. C C H H H H Ethene C C H H H C H H H Propene The arrangement of bonds around the >C=C< is planar and has the bond angle 120o Numbers need to be added to the name when positional isomers can occur. C H H C C C H H H H H H But-1-ene But-2-ene C=C double covalent bond consists of one sigma (σ) bond and one pi (π) bond. π bonds are exposed and have high electron density. They are therefore vulnerable to attack by species which ‘like’ electrons: these species are called electrophiles.
3.3.4.1 Structure, bonding and reactivity
Alkenes are unsaturated hydrocarbons.
Bonding in alkenes involves a double covalent bond, a centre of high electron density.
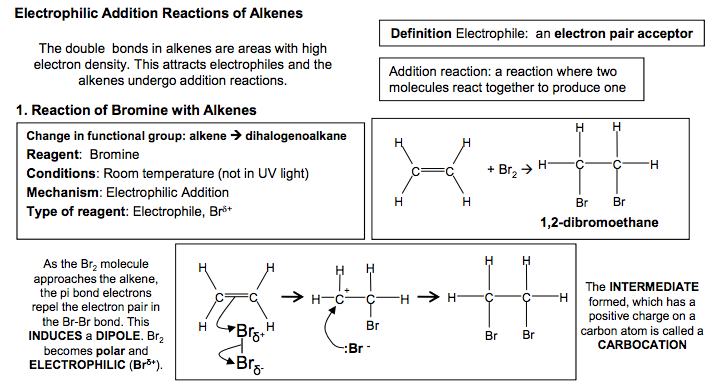
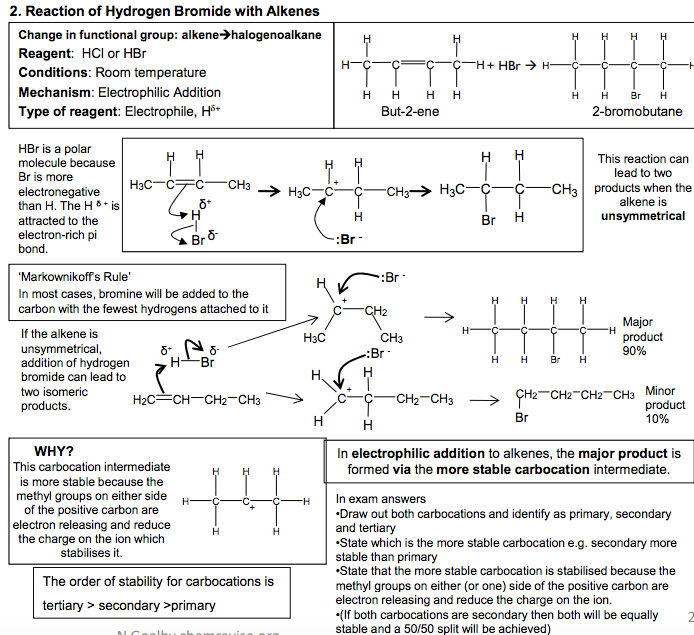
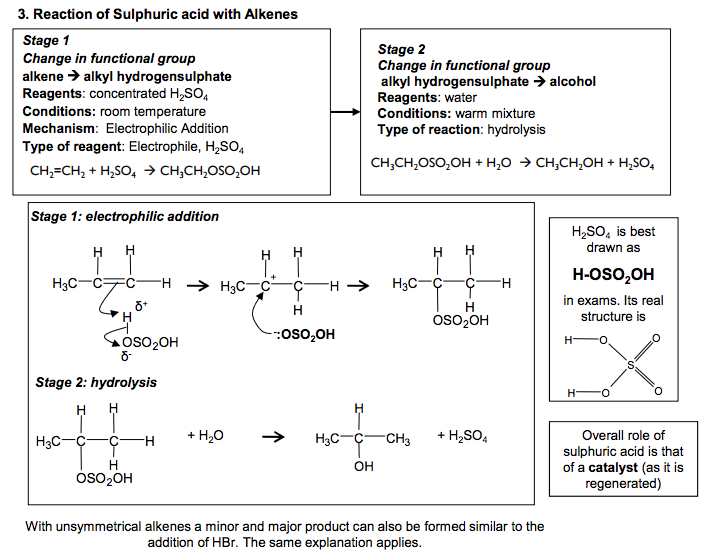
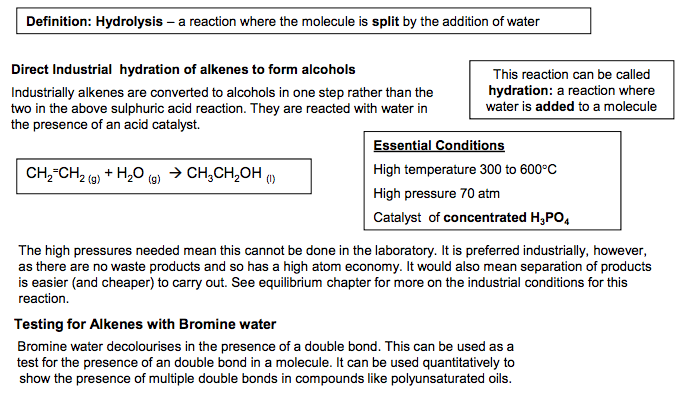
Electrophilic Addition Reactions of Alkenes Definition Electrophile: an electron pair acceptor The double bonds in alkenes are areas with high electron density. This attracts electrophiles and the alkenes undergo addition reactions. Addition reaction: a reaction where two molecules react together to produce one 1. Reaction of Bromine with Alkenes Change in functional group: alkene dihalogenoalkane Reagent: Bromine Conditions: Room temperature (not in UV light) Mechanism: Electrophilic Addition Type of reagent: Electrophile, Br+ C C H H Br Br H H C C H H H H + Br2 1,2-dibromoethane As the Br2 molecule approaches the alkene, the pi bond electrons repel the electron pair in the Br-Br bond. This INDUCES a DIPOLE. Br2 becomes polar and ELECTROPHILIC (Brδ+ ). The INTERMEDIATE formed, which has a positive charge on a carbon atom is called a CARBOCATION 2. Reaction of Hydrogen Bromide with Alkenes Change in functional group: alkenehalogenoalkane Reagent: HCl or HBr Conditions: Room temperature Mechanism: Electrophilic Addition Type of reagent: Electrophile, H+ C + HBr H H C C C H H H H H H C C H C H Br C H H H H H H H But-2-ene 2-bromobutane HBr is a polar molecule because Br is more electronegative than H. The H δ + is attracted to the electron-rich pi bond. This reaction can lead to two products when the alkene is unsymmetrical Major product 90% Minor product 10% If the alkene is unsymmetrical, addition of hydrogen bromide can lead to two isomeric products. ‘Markownikoff’s Rule’ In most cases, bromine will be added to the carbon with the fewest hydrogens attached to it C C H H H C H H H H + This carbocation intermediate is more stable because the methyl groups on either side of the positive carbon are electron releasing and reduce the charge on the ion which stabilises it. WHY? The order of stability for carbocations is tertiary > secondary >primary In electrophilic addition to alkenes, the major product is formed via the more stable carbocation intermediate. In exam answers •Draw out both carbocations and identify as primary, secondary and tertiary •State which is the more stable carbocation e.g. secondary more stable than primary •State that the more stable carbocation is stabilised because the methyl groups on either (or one) side of the positive carbon are electron releasing and reduce the charge on the ion. •(If both carbocations are secondary then both will be equally stable and a 50/50 split will be achieved) C C H H H H C C H H Br Br H H C + C Br H H H H Br Br δ +δ – :Br – C + C H CH3 H H H3C C C H CH3 H H H3C Br C C CH3 H H H3C δ +δ – :Br – H Br H2C CH CH2 CH3 H Br H3C C + CH2 CH3 H C + C CH2 CH3 H H H H :Br – :Br δ – + δ – CH2 CH2 CH2 CH3 Br C C H C H Br C H H H H H H H 2 N Goalby chemrevise.org H OSO2OH 3. Reaction of Sulphuric acid with Alkenes Stage 1 Change in functional group alkene alkyl hydrogensulphate Reagents: concentrated H2SO4 Conditions: room temperature Mechanism: Electrophilic Addition Type of reagent: Electrophile, H2SO4 CH2=CH2 + H2SO4 CH3CH2OSO2OH CH3CH2OSO2OH + H2O CH3CH2OH + H2SO4 Stage 2 Change in functional group alkyl hydrogensulphate alcohol Reagents: water Conditions: warm mixture Type of reaction: hydrolysis H2SO4 is best drawn as H-OSO2OH in exams. Its real structure is Stage 1: electrophilic addition Stage 2: hydrolysis S O O O O H H With unsymmetrical alkenes a minor and major product can also be formed similar to the addition of HBr. The same explanation applies. Testing for Alkenes with Bromine water Bromine water decolourises in the presence of a double bond. This can be used as a test for the presence of an double bond in a molecule. It can be used quantitatively to show the presence of multiple double bonds in compounds like polyunsaturated oils. Direct Industrial hydration of alkenes to form alcohols Industrially alkenes are converted to alcohols in one step rather than the two in the above sulphuric acid reaction. They are reacted with water in the presence of an acid catalyst. CH2=CH2 (g) + H2O (g) CH3CH2OH (l) This reaction can be called hydration: a reaction where water is added to a molecule Essential Conditions High temperature 300 to 600°C High pressure 70 atm Catalyst of concentrated H3PO4 The high pressures needed mean this cannot be done in the laboratory. It is preferred industrially, however, as there are no waste products and so has a high atom economy. It would also mean separation of products is easier (and cheaper) to carry out. See equilibrium chapter for more on the industrial conditions for this reaction. Overall role of sulphuric acid is that of a catalyst (as it is regenerated) Definition: Hydrolysis – a reaction where the molecule is split by the addition of water C C H H H H3C δ + δ – -:OSO2OH C + C H H H H3C H C C H H H H3C H OSO2OH C C H H H H3C H OSO2OH + H2O C CH3 + H2SO4 H H3C OH N Goalby chemrevise.org
3.3.4.2 Addition reactions of alkenes
Electrophilic addition reactions of alkenes with HBr, H2 SO4 and Br2
The use of bromine to test for unsaturation.
The formation of major and minor products in addition reactions of unsymmetrical alkenes.
Students should be able to:
• outline the mechanisms for these reactions
• explain the formation of major and minor products by reference to the relative stabilities of primary, secondary and tertiary carbocation intermediates.

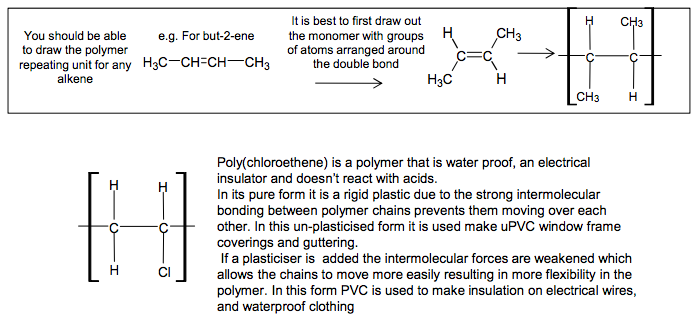
Addition Polymers Addition polymers are formed from alkenes Poly(alkenes) like alkanes are unreactive due to the strong C-C and C-H bonds C C H H CH3 H propene n poly(propene) C C C C C C CH3 H H H CH3 H H H CH3 H H H be able to recognise the repeating unit in a poly(alkene) Poly(propene) is recycled This is called addition polymerisation H3C CH CH CH3 H3C C C CH3 H You should be able H to draw the polymer repeating unit for any alkene It is best to first draw out the monomer with groups of atoms arranged around the double bond e.g. For but-2-ene 4 If asked to draw one repeating unit, don’t add the n on to your diagram, because n represents a large number Add the n’s if writing an equation showing the reaction where ‘n’ monomers become ‘n’ repeating units C C H CH3 H H C C CH3 H H CH3 n N Goalby chemrevise.org C C H CH3 H H C C H Cl H H Poly(chloroethene) is a polymer that is water proof, an electrical insulator and doesn’t react with acids. In its pure form it is a rigid plastic due to the strong intermolecular bonding between polymer chains prevents them moving over each other. In this un-plasticised form it is used make uPVC window frame coverings and guttering. If a plasticiser is added the intermolecular forces are weakened which allows the chains to move more easily resulting in more flexibility in the polymer. In this form PVC is used to make insulation on electrical wires, and waterproof clothing
3.3.4.3 Addition polymers
Addition polymers are formed from alkenes and substituted alkenes.
The repeating unit of addition polymers.
IUPAC rules for naming addition polymers.
Addition polymers are unreactive.
Appreciate that knowledge and understanding of the production and properties of polymers has developed over time.
Typical uses of poly(chloroethene), commonly known as PVC, and how its properties can be modified using a plasticiser.
Students should be able to:
• draw the repeating unit from a monomer structure
• draw the repeating unit from a section of the polymer chain
• draw the structure of the monomer from a section of the polymer
• explain why addition polymers are unreactive
• explain the nature of intermolecular forces between molecules of polyalkenes.








