Physical and chemical properties


Alkanes and cycloalkanes are saturated hydrocarbons Remember that the shape around the carbon atom in saturated hydrocarbons is tetrahedral and the bond angle is 109.5O The shape is tetrahedral as a result of the four bond pairs of electrons equally repelling. C C H H H H H H Boiling Point The increasing boiling points of the alkane homologous series can be explained by the increasing number of electrons in the bigger molecules causing an increase in the size of the induced dipole–dipole interactions (London forces) between molecules. The shape of the molecule can also have an effect on the size of the induced dipole–dipole interactions (London forces) . Long chain alkanes have a larger surface area of contact between molecules for London force to form than compared to spherical shaped branched alkanes and so have stronger induced dipole– dipole interactions and higher boiling points. One sp2 orbital from each carbon overlap to form a single C-C bond called a sigma σ bond C C sigma σ bond Formation of σ bond Rotation can occur around a sigma bond Reactivity The low reactivity of alkanes with many reagents can be explained by the high bond enthalpies of the C-C and C-H bonds and the very low polarity of the σ-bonds present.
/
~
~
~
/
4.1.2 Alkanes

Properties of alkanes (a) alkanes as saturated hydrocarbons containing single C–C and C–H bonds as σ-bonds (overlap of orbitals directly between the bonding atoms); free rotation of the σ-bond Hybridisation not required. HSW1 Use of model of orbital overlap to explain covalent bonding in organic compounds. (b) explanation of the tetrahedral shape and bond angle around each carbon atom in alkanes in terms of electron pair repulsion (see also 2.2.2 g–h) M4.1, M4.2 Learners should be able to draw 3-D diagrams. (c) explanation of the variations in boiling points of alkanes with different carbon-chain length and branching, in terms of induced dipole–dipole interactions (London forces) (see also 2.2.2 k) M3.1 Reactions of alkanes (d) the low reactivity of alkanes with many reagents in terms of the high bond enthalpy and very low polarity of the σ-bonds present (see also 2.2.2 j) HSW1 Use of ideas about enthalpy and polarity to explain macroscopic properties of alkanes.
Combustion
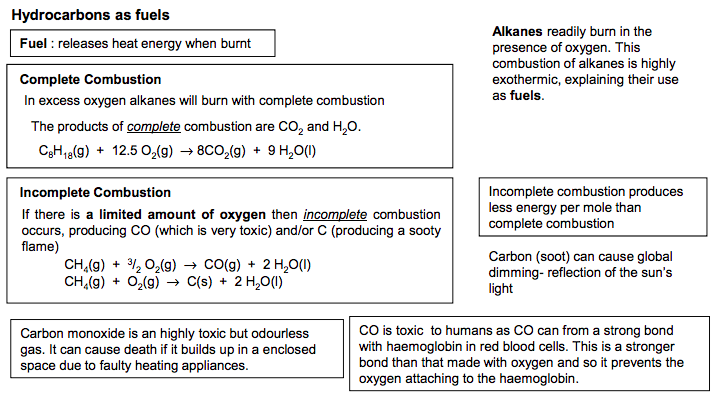
Alkanes readily burn in the presence of oxygen. This combustion of alkanes is highly exothermic, explaining their use as fuels. Complete Combustion C8H18(g) + 12.5 O2 (g) → 8CO2 (g) + 9 H2O(l) Fuel : releases heat energy when burnt Incomplete combustion produces less energy per mole than complete combustion If there is a limited amount of oxygen then incomplete combustion occurs, producing CO (which is very toxic) and/or C (producing a sooty flame) CH4 (g) + 3 / 2 O2 (g) → CO(g) + 2 H2O(l) CH4 (g) + O2 (g) → C(s) + 2 H2O(l) The products of complete combustion are CO2 and H2O. In excess oxygen alkanes will burn with complete combustion Incomplete Combustion Carbon (soot) can cause global dimming- reflection of the sun’s light Hydrocarbons as fuels CO is toxic to humans as CO can from a strong bond with haemoglobin in red blood cells. This is a stronger bond than that made with oxygen and so it prevents the oxygen attaching to the haemoglobin. Carbon monoxide is an highly toxic but odourless gas. It can cause death if it builds up in a enclosed space due to faulty heating appliances.
/
~
~
~
/
4.1.2 Alkanes
(e) complete combustion of alkanes, as used in fuels, and the incomplete combustion of alkane fuels in a limited supply of oxygen with the resulting potential dangers from CO
Free radical substitution

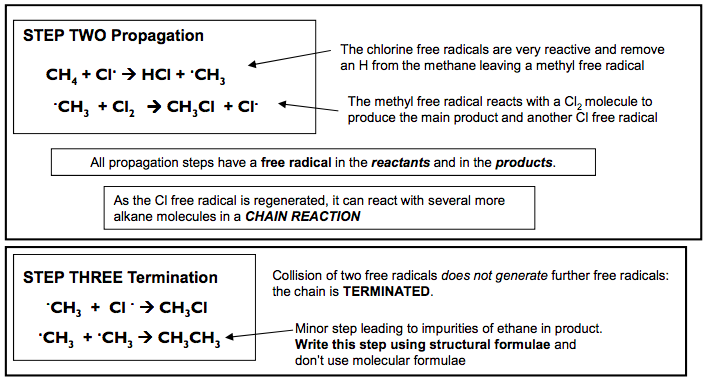

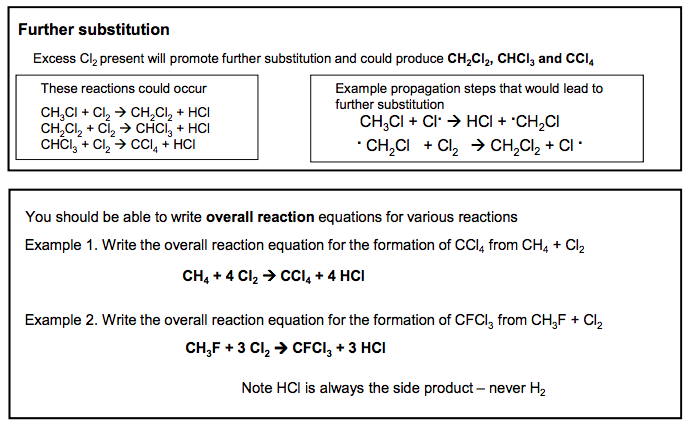
Substitution reactions of alkanes Reaction of alkanes with bromine / chlorine in UV light In the presence of UV light alkanes react with chlorine to form a mixture of products with the halogens substituting hydrogen atoms. In general, alkanes do not react with many reagents. This is because the C-C bond and the C-H bond are relatively strong Overall Reaction CH4 + Cl2 CH3Cl + HCl methane chloromethane This is the overall reaction, but a more complex mixture of products is actually formed To understand this reaction fully we must look in detail at how it proceeds step by step. This is called its mechanism The MECHANISM for this reaction is called a FREE RADICAL SUBSTITUTION It proceeds via a series of steps: STEP ONE: Initiation STEP TWO: Propagation STEP THREE: Termination STEP ONE Initiation Cl2 2Cl. Essential condition: UV light The UV light supplies the energy to break the Cl-Cl bond. It is broken in preference to the others as it is the weakest. The bond has broken in a process called homolytic fission. each atom gets one electron from the covalent bond When a bond breaks by homolytic fission it forms Free Radicals. Free Radicals do not have a charge and are represented by a DEFINITION A Free Radical is a reactive species which possess an unpaired electron CH4 + Cl. HCl + .CH3 STEP TWO Propagation .CH3 + Cl2 CH3Cl + Cl. The chlorine free radicals are very reactive and remove an H from the methane leaving a methyl free radical The methyl free radical reacts with a Cl2 molecule to produce the main product and another Cl free radical All propagation steps have a free radical in the reactants and in the products. As the Cl free radical is regenerated, it can react with several more alkane molecules in a CHAIN REACTION STEP THREE Termination .CH3 + Cl . CH3Cl .CH3 + .CH3 CH3CH3 Collision of two free radicals does not generate further free radicals: the chain is TERMINATED. Minor step leading to impurities of ethane in product. Write this step using structural formulae and don’t use molecular formulae N Goalby chemrevise.org 3 Applying the mechanism to other alkanes The same mechanism is used: Learn the patterns in the mechanism STEP ONE Initiation Br2 2Br . Essential condition: UV light Example: Write mechanism of Br2 and Propane Br2 splits in the same way as Cl2 CH3CH2CH3 + Br. HBr + CH3CH2CH2 . STEP TWO Propagation CH3CH2CH2 . + Br2 CH3CH2CH2Br + Br. Remove one H from the alkane to produce a radical To the radical produced in the previous step add a Br STEP THREE Termination CH3CH2CH2 . + Br. CH3CH2CH2Br CH3CH2CH2 . + CH3CH2CH2 . CH3CH2CH2CH2CH2CH3 4 N Goalby chemrevise.org Further substitution Excess Cl2 present will promote further substitution and could produce CH2Cl2 , CHCl3 and CCl4 These reactions could occur CH3Cl + Cl2 CH2Cl2 + HCl CH2Cl2 + Cl2 CHCl3 + HCl CHCl3 + Cl2 CCl4 + HCl CH3Cl + Cl. HCl + .CH2Cl . CH2Cl + Cl2 CH2Cl2 + Cl . Example propagation steps that would lead to further substitution You should be able to write overall reaction equations for various reactions Example 1. Write the overall reaction equation for the formation of CCl4 from CH4 + Cl2 CH4 + 4 Cl2 CCl4 + 4 HCl Example 2. Write the overall reaction equation for the formation of CFCl3 from CH3F + Cl2 CH3F + 3 Cl2 CFCl3 + 3 HCl Note HCl is always the side product – never H2
/
~
~
~
/
4.1.2 Alkanes
(f) the reaction of alkanes with chlorine and bromine by radical substitution using ultraviolet radiation, including a mechanism involving homolytic fission and radical reactions in terms of initiation, propagation and termination (see also 4.1.1 f–g) Learners are not required to use ‘half curly arrows’ in this mechanism. Equations should show which species are radicals using a single ‘dot’, •, to represent the unpaired electron. (g) the limitations of radical substitution in synthesis by the formation of a mixture of organic products, in terms of further substitution and reactions at different positions in a carbon chain.
Credits: Neil Goalby

